
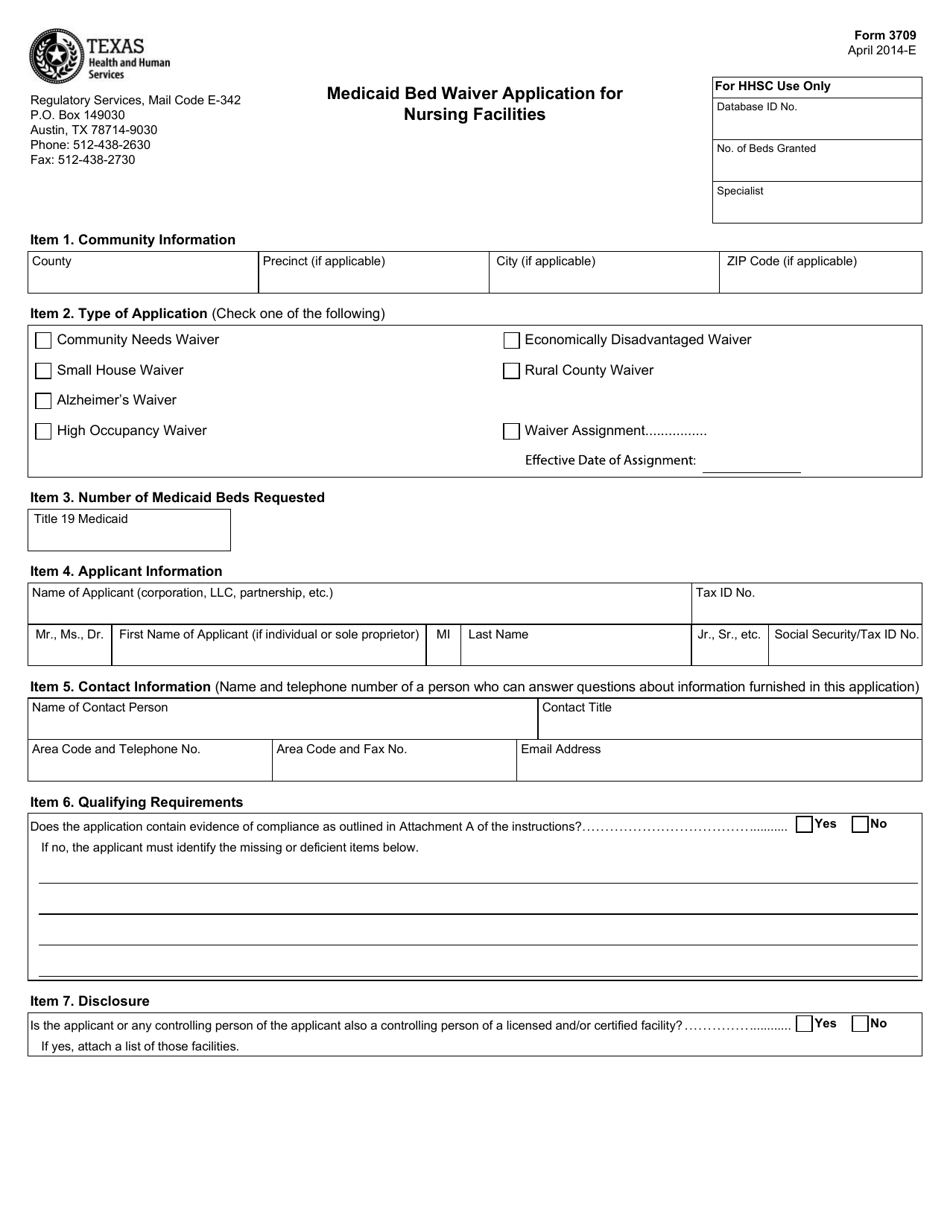
Xenazine (tetrabenazine) is an FDA-approved medicine used to treat involuntary movements (chorea) caused by Huntington's disease, which is a rare, inherited disease that causes nerve cells in the brain to breakdown and die. I f I cancel, Ovation will cease using or disclosing my information for the purposes listed above, except as required by law or as necessary for the orderly termination of my participation in the Xenazine Information Center.


Proper use of Xenazine requires attention to all facets of the underlying disease process over time (see CLINICAL STUDIES in Xenazine1-88. Whether these effects persist, resolve, or worsen with continued treatment is unknown. Xenazine was also shown to cause slight worsening in mood, cognition, rigidity, and functional capacity. As …Xenazine - Uses, Side Effects, and More Generic Name: tetrabenazine Tetrabenazine is used to decrease the uncontrollable movements (chorea) caused by Huntington's disease. rhino pills ingredients Xenazine (tetrabenazine) is a brand-name oral tablet that’s prescribed for chorea, a movement condition that can happen in people with Huntington’s disease. XENAZINE) zolmitriptan tetracycline TEXACORT SOLUTION THEO-24 theophylline ext-rel tablet zonisamide thiothixene valacyclovir tiagabine valganciclovir timolol maleate solution ZYLET tinidazole valproic acid tizanidine tablet valsartan tobramycin X valsartan tobramycin inhalation solution (generic KITABIS, TOBI) SGM tobramycin-dexamethasone.
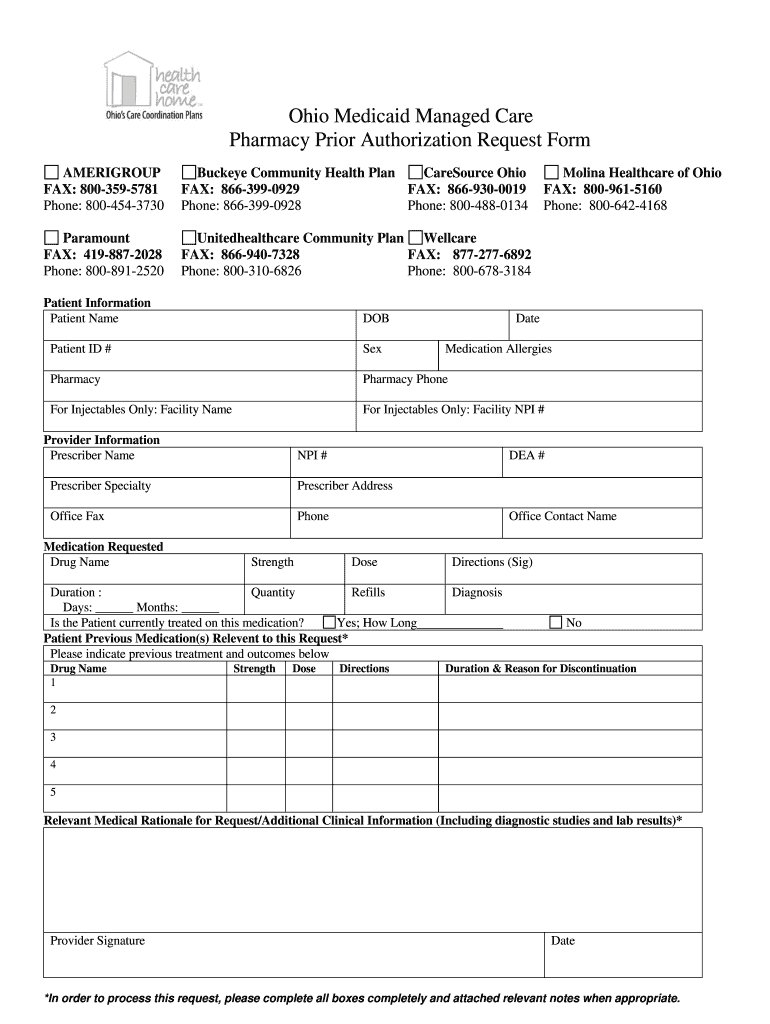
Xenazine does not cure the cause of the involuntary movements, and it does not treat other symptoms of Huntington’s disease, such as problems with thinking or emotions.


 0 kommentar(er)
0 kommentar(er)
